Modern bioprocessing and biosafety laboratories face an increasingly complex challenge: maintaining absolute containment while ensuring operational efficiency. As regulatory standards tighten and contamination risks escalate, facilities require filtration solutions that deliver continuous protection without compromising workflow productivity. IN SITU PIPELINE HEPA and DOUBLE IN SITU PIPELINE HEPA systems address these critical operational demands through integrated filtration technology that enables testing, maintenance, and disinfection without system shutdown or containment breach.
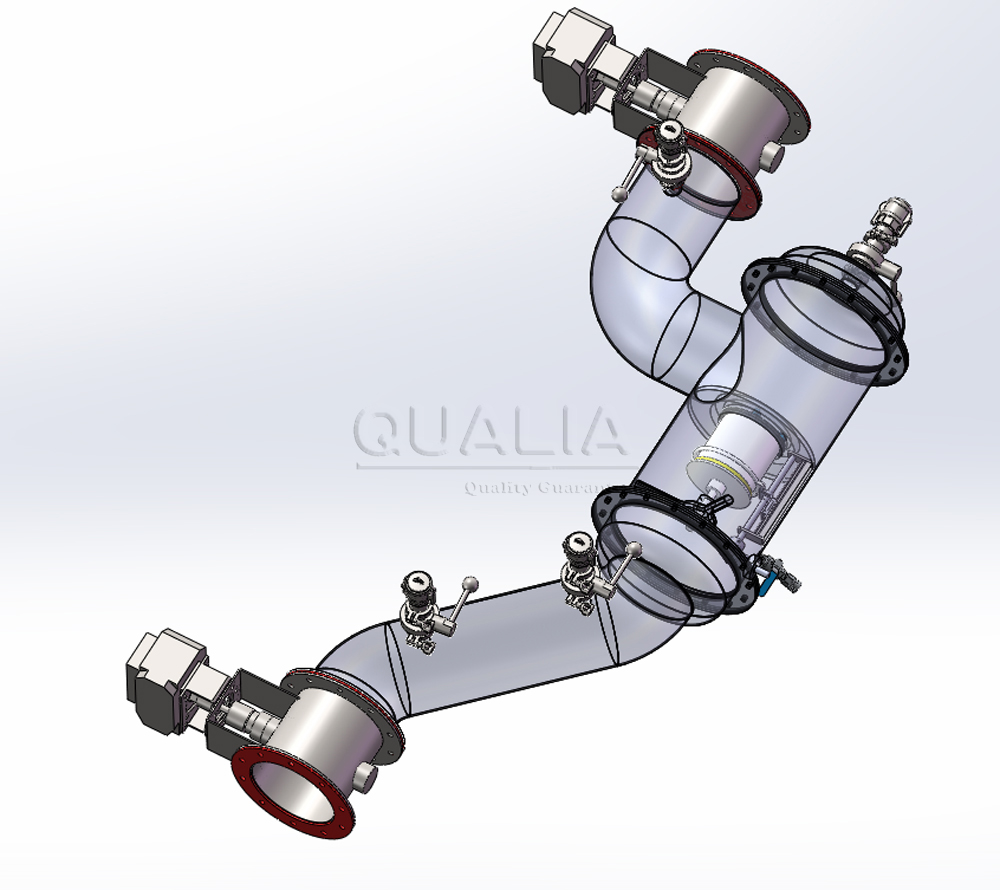
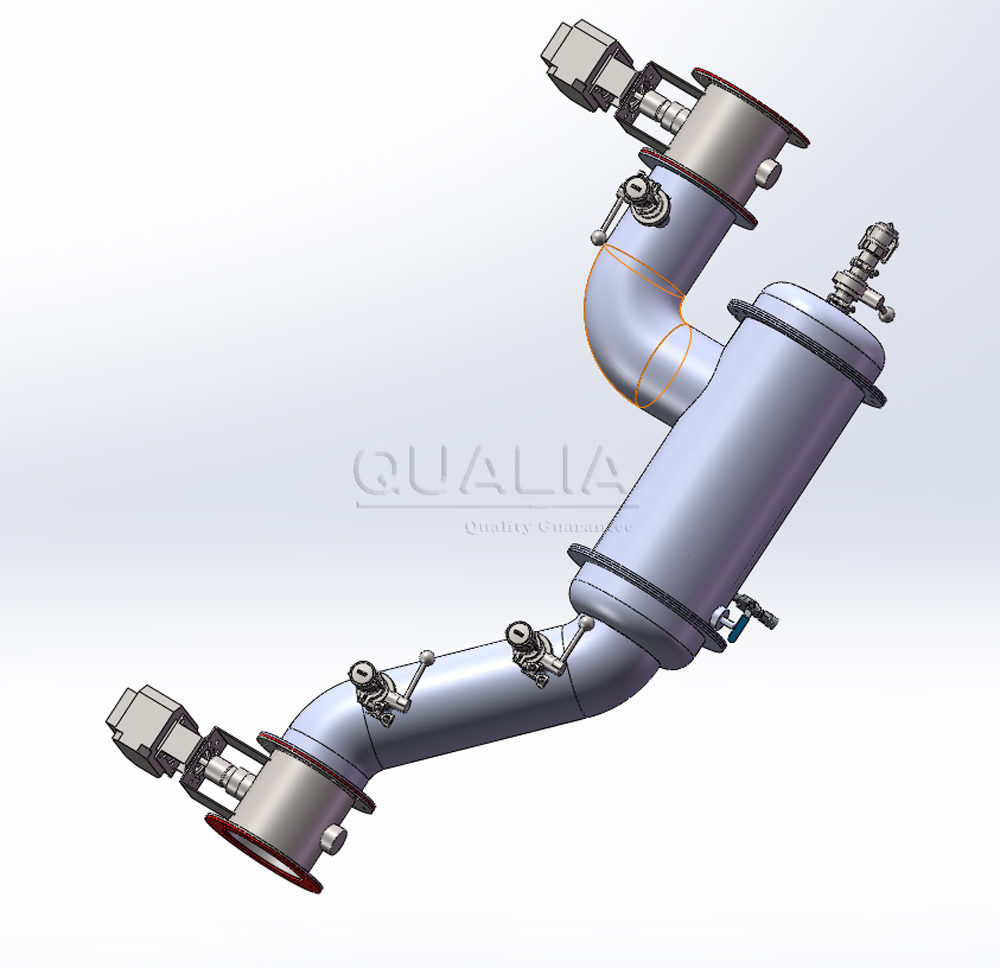
Understanding In Situ Pipeline HEPA Filtration Systems
What These Systems Deliver
IN SITU PIPELINE HEPA systems represent a fundamental shift from traditional filtration approaches. Rather than requiring system shutdown for testing or maintenance, these integrated solutions enable all critical operations—including leak detection, disinfection, and filter replacement—while maintaining containment integrity. The “in situ” designation refers to the system’s capability to perform these functions in place, without removing components from their operational environment.
Core Functionality & Design Approach
These systems integrate multiple critical functions within a single, sealed housing. The design typically includes a static pressure body constructed from stainless steel 304/316L, high-efficiency filter elements, centralized interface controls, and automated scanning capabilities. The centralized interface box consolidates all operational controls—scanning handles, testing ports, and disinfection connections—into a single access point, minimizing contamination risks during routine operations.
Double In Situ Pipeline HEPA Configuration
The double configuration provides enhanced protection through redundant filtration stages. This approach proves particularly valuable in BSL-3 and BSL-4 environments where single-point failures could compromise entire operations. Each filtration stage operates independently, allowing individual testing and maintenance while the system continues operation through the secondary stage.
Industry experience indicates that facilities using double in situ configurations typically achieve 99.9% uptime compared to 85-90% with conventional systems requiring shutdown for maintenance.
Applications Across Critical Environments
These systems address filtration needs in wastewater treatment lines, exhaust streams, and pipeline applications where traditional HEPA installations prove inadequate. Bioprocessing facilities use them for final product filtration before filling, while research laboratories employ them for exhaust air treatment from containment areas.
▶ Request Technical Documentation ◀
Technical Specifications & Performance Characteristics
| Specification Category | Single In Situ Pipeline | Double In Situ Pipeline |
|---|---|---|
| Filtration Efficiency | ≥99.99% at 0.3μm | ≥99.99% per stage at 0.3μm |
| Pressure Resistance | ±2500Pa (10 minutes) | ±2500Pa (10 minutes) |
| Airtightness | ≤0.1% leakage/minute at 1000Pa | ≤0.05% leakage/minute at 1000Pa |
| Initial Resistance | ≤250Pa at rated volume | ≤300Pa per stage |
| Materials | SS304/316L, fully welded | SS304/316L, fully welded |
| Testing Capability | Manual scanning detection | Automated scanning per stage |
Integration & Compatibility Features
Modern in situ pipeline HEPA systems accommodate various installation configurations. Ceiling and sidewall mounting options provide flexibility for space-constrained environments. The centralized interface design enables integration with existing facility management systems while maintaining independent operational capability.
Performance Monitoring & Validation
Built-in differential pressure monitoring provides continuous performance indication. The integrated scanning detection systems meet IEST RP-CC-001.4 requirements for laminar flow and biological grade HEPA filters, enabling compliance verification without external testing equipment.
| Performance Metric | Standard Range | Double Configuration Advantage |
|---|---|---|
| Operational Uptime | 85-90% | 99%+ |
| Testing Frequency | Weekly shutdown required | Continuous online testing |
| Maintenance Windows | 4-8 hours | 30-60 minutes |
| Contamination Risk | Medium (exposure during changes) | Minimal (sealed operations) |
Real-World Implementation Outcomes
Pharmaceutical Manufacturing Facility Results
A medical device manufacturer implementing 1,000 MEGAcel II eFRM HEPA filters achieved remarkable results. The installation process completed in 12 days with zero filter damage during handling and installation. More significantly, the facility achieved 100% filter success rate with 0% failure incidents through the first operational period, compared to previous glass filter installations that required maintaining 10% spare inventory due to handling damage.
The implementation delivered additional operational benefits beyond reliability. The facility reduced fresh air intake dampers from 100% to 20% open, significantly reducing HVAC system load and extending equipment lifetime. Energy savings materialized through multiple pathways, including reduced pressure drop requirements and improved system stability.
Bioprocessing Laboratory Integration
Research facilities implementing in situ filtration technology report substantial improvements in operational efficiency. One implementation reduced certification downtime by over 60% through integrated photometric sensors that perform automated integrity tests during operational pauses, eliminating the need for specialized aerosol generation equipment and external testing protocols.
Biosafety Laboratory Applications
BSL-3 and BSL-4 facilities using double in situ pipeline HEPA systems for wastewater treatment report enhanced safety profiles and simplified compliance management. The ability to perform leak detection and disinfection without containment breach reduces operator exposure risks while maintaining regulatory compliance requirements.
▶ Discuss Your Specific Requirements ◀
Implementation Strategy & Support Framework
Typical Implementation Timeline
Most installations follow a structured 4-6 week implementation process. Initial assessment and planning occupy the first week, including spatial analysis and integration planning. Equipment procurement and staging require 2-3 weeks depending on customization requirements. Installation and commissioning typically complete within 5-7 days for standard configurations.
Integration Considerations
Successful implementation requires careful attention to existing infrastructure compatibility. Most in situ filtration systems accommodate standard bioreactor connections, but verification of specific dimensions and interfaces proves essential before procurement. Control system integration often requires IT support, particularly for systems incorporating data logging or network connectivity capabilities.
Training & Expertise Development
While basic operation remains straightforward, developing optimization expertise requires comprehensive training programs. Manufacturers typically provide application specialists for initial setup and protocol development. This support proves invaluable for establishing application-specific procedures and troubleshooting initial challenges.
Validation Requirements
GMP environments require specific validation protocols. While in situ filtration can simplify some validation aspects by eliminating transfer steps, the integrated technology may require revised validation approaches. Early consultation with quality assurance personnel ensures appropriate documentation from implementation outset.
| Implementation Phase | Duration | Key Activities | Support Requirements |
|---|---|---|---|
| Assessment | 1 week | Site survey, compatibility verification | Technical consultation |
| Procurement | 2-3 weeks | Equipment customization, staging | Project management |
| Installation | 5-7 days | Physical installation, integration | Certified technicians |
| Commissioning | 3-5 days | Testing, calibration, validation | Application specialists |
| Training | 1-2 weeks | Operations, maintenance, optimization | Technical training team |
▶ Schedule Implementation Consultation ◀
Frequently Asked Questions
How do in situ testing capabilities compare to traditional shutdown methods?
In situ testing eliminates the 4-8 hour shutdown windows typically required for HEPA filter integrity verification. The integrated scanning systems meet ISO 14644-3 standards while enabling testing during operational pauses rather than full system shutdown. This approach reduces testing-related downtime by 80-90% while maintaining compliance with regulatory requirements.
What maintenance advantages do double in situ configurations provide?
Double configurations enable maintenance of one filtration stage while maintaining operation through the secondary stage. This approach virtually eliminates maintenance-related downtime while ensuring continuous protection. Filter replacement procedures can be scheduled during normal operational pauses rather than requiring dedicated shutdown windows.
How do these systems address biocontainment requirements?
The centralized interface design minimizes contamination risks during routine operations. All testing, disinfection, and maintenance functions operate through sealed interfaces, maintaining containment integrity throughout operational and maintenance cycles. This approach proves particularly valuable in BSL-3 and BSL-4 environments where containment breach risks must be minimized.
What are typical return on investment timeframes?
Most facilities achieve ROI within 12-18 months through reduced downtime, lower maintenance costs, and improved operational efficiency. Energy savings from reduced pressure drop requirements contribute additional value, with some implementations achieving 40% reductions in filtration-related energy consumption.
How do implementation costs compare to traditional HEPA installations?
While initial equipment costs typically run 20-30% higher than conventional systems, the reduced operational costs and improved reliability deliver net savings within the first operational year. The elimination of testing-related shutdown costs alone often justifies the implementation investment.
▶ Connect with Technical Team ◀
Market Position & Technology Landscape
| Solution Category | Upfront Cost | Operational Efficiency | Maintenance Requirements | Compliance Support |
|---|---|---|---|---|
| Traditional HEPA | Low | 75-85% | High (shutdown required) | Manual verification |
| Standard In Situ | Medium | 90-95% | Medium (reduced downtime) | Automated testing |
| Double In Situ | High | 95-99% | Low (redundant operation) | Continuous verification |
| Custom Solutions | Variable | Variable | Variable | Application-specific |
Competitive Differentiation
The in situ pipeline HEPA market includes several established players, each with specific strengths. American Air Filter Company (AAF) focuses on large-scale cleanroom applications, while Camfil emphasizes healthcare and nuclear industry solutions. W.L. Gore & Associates specializes in high-volume industrial emissions control.
The QUALIA approach differentiates through integrated European technology partnerships and specialized focus on bioprocessing applications. The emphasis on BSL-3/BSL-4 compliance and wastewater treatment applications addresses specific market segments where conventional solutions prove inadequate.
Market Evolution & Industry Trends
The global HEPA filters market continues expanding, with projections indicating growth from USD 3.89 billion in 2024 to USD 6.13 billion by 2033. This growth reflects increasing awareness of air quality requirements and expanding bioprocessing activities, particularly in pharmaceutical and biotechnology sectors.
Selection Criteria for Procurement Teams
Successful system selection requires balancing multiple factors: initial investment costs, operational efficiency gains, maintenance requirements, and compliance support capabilities. Organizations should evaluate total cost of ownership over 3-5 year periods rather than focusing solely on acquisition costs.
The most critical selection factor often involves long-term scalability and support capabilities. As bioprocessing requirements evolve and regulatory standards change, systems must accommodate modifications and upgrades without requiring complete replacement.
For facilities handling hazardous materials or operating under stringent regulatory requirements, the enhanced safety profile and simplified compliance management of in situ systems typically outweigh initial cost considerations. The ability to maintain operations while ensuring safety and compliance delivers value that extends far beyond traditional cost-benefit calculations.
QUALIA Bio-Tech continues advancing in situ filtration technology through ongoing research partnerships and customer feedback integration, ensuring these systems evolve with changing industry requirements and emerging bioprocessing challenges.
Related Contents:
- In Situ vs Ex Situ Filtration: Which is Right for You?
- In Situ Filtration vs Batch Filtration: A Comparison
- Optimizing Biotech Processes with In Situ Filtration
- Boost Productivity: In Situ Filtration Efficiency
- The Definitive Guide to Selecting In Situ Filters
- The Ultimate Guide to In Situ Filtration Systems
- 5 Strategies for Scaling Up In Situ Filtration Systems
- 5 Ways In Situ Filtration Enhances Process Safety
- Top 5 Industrial Applications for In Situ Filtration































