High-Performance Biopharmaceutical Cleanroom Equipment & Systems
QUALIA delivers advanced biopharmaceutical cleanroom equipment designed to meet the most stringent safety and regulatory requirements in the industry. Our comprehensive range of solutions includes specialized containment systems for BSL-3 and BSL-4 laboratories, integrating cutting-edge technology with practical designs that enhance both safety and operational efficiency. With decades of expertise in cleanroom infrastructure and OEB protective measures, we provide pharmaceutical and biotechnology companies with reliable equipment that ensures complete protection throughout critical manufacturing processes.
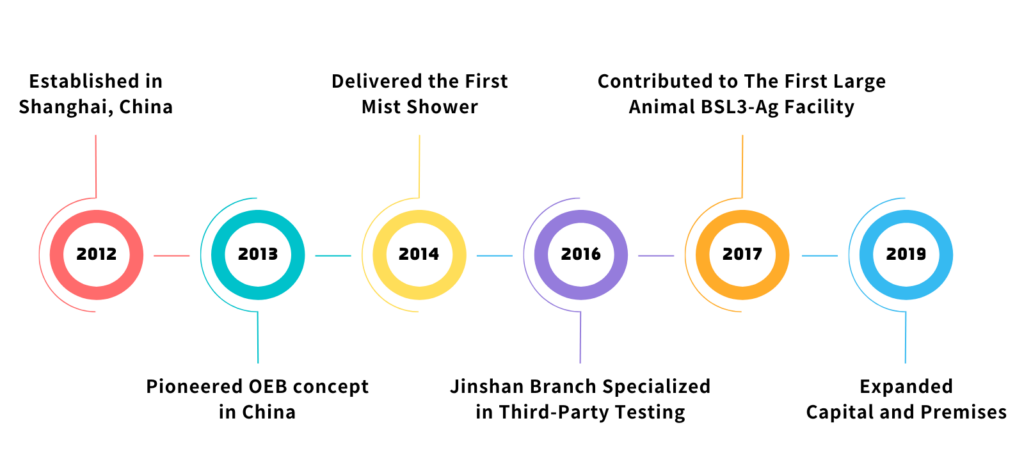
Collaborative Design and
Construction of China’s First BSL3-Ag Large Animal Room.
Provided Core Services to 70+ Global BSL3 Laboratories & Vaccine Production Workshops.
A Global Network of 210+ Clients, Empowered by Strategic Collaborative Partnerships.
Provided over 1800 APR Doors, 930 Mist Showers, 850 BIBO, and 400 VHP Passboxes.
Biopharmaceutical Cleanroom Validation Requirements
Implementing proper biopharmaceutical cleanroom validation requirements is essential for maintaining regulatory compliance and product integrity. Our validation protocols ensure your cleanroom equipment meets stringent industry standards while supporting operational efficiency.
Advanced R&D
Leading-edge products developed by a global team of experts.
Trusted Brand
Long-standing relationships with a loyal customer base in the pharmaceutical and healthcare sectors.
Expertise in Protection
Extensive experience with P3, P4 lab standards and OEB industry regulations.
One-stop Solution
Complete turnkey projects for clean rooms and associated equipment.
Capacity of QUALIA
Cleanroom Equipments & Solutions
Air Series
In Situ Filtration System
ISO Series
cRABS
OEB4/5 Isolator
Biosafety Isolator
Lock Series
Bio-safety lsolation Damper
Mechanical Seal APR Doors
Pneumatic Seal APR Doors
Module Laboratory
Mobile Module Laboratory
Pass Series
Biosafety Dunk Tank
VHP Pass Box
Biosafety Pass Box
Shower Series
Mist Shower
Chemical Shower
Water Shower
Lock Series
VHP Generator Type I
VHP Generator Type II III
Water Treatment
In Situ Pipeline HEPA
Bag-In/Bag-Out - BIBO Systems in BSL-3/BSL-4 Environments

A typical BIBO filter change involves sealing the outer bag to the housing, removing the used filter into the inner bag, and then securely sealing and removing the bagged filter for safe disposal. This process is supported by glove ports and clear SOPs, minimizing human error and exposure risk. The result is a validated, repeatable procedure that meets or exceeds international biosafety and engineering standards such as ISO 14644, ASME AG-1, and IEST-RP-CC001.
Comprehensive Containment Solutions for Biopharmaceutical Manufacturing
Biopharmaceutical manufacturing demands uncompromising control of airborne particulates, cross-contamination, and occupational exposure. Comprehensive containment solutions integrate multiple layers of engineering controls, including BIBO systems, high-efficiency particulate air (HEPA/ULPA) filtration, negative pressure zones, and custom isolators. These systems are designed to protect both product integrity and personnel safety throughout the entire process chain—from raw material handling to final product packaging.
These solutions are engineered to comply with global standards such as EU GMP Annex 1, FDA 21 CFR Part 211, and ISO 14644. The result is a manufacturing environment that supports regulatory inspections, reduces the risk of batch loss, and enhances overall productivity.
IOS 14644
Cleanroom Standard
BIBO filtration units for all critical exhaust and supply air streams, ensuring safe filter maintenance and validated containment.
Custom isolators and barrier systems for aseptic processing, API handling, and potent compound management.
Pressure cascade management to maintain directional airflow and prevent cross-contamination between clean and dirty zones.
Continuous environmental monitoring for real-time detection of particle counts, pressure deviations, and system performance.
Validated decontamination protocols for equipment and facility surfaces, supporting rapid changeover and minimizing downtime.
OEB Protective Measures and Safety Standards
Occupational Exposure Band (OEB) protective measures are essential for safeguarding personnel working with high-potency active pharmaceutical ingredients (HPAPIs) and hazardous compounds. Modern containment solutions are designed to meet strict OEB requirements, providing layered protection through engineering controls, validated processes, and continuous monitoring.
Key elements of OEB protective measures include:
Closed-system transfer devices and glovebox isolators to eliminate direct operator contact with hazardous materials.
BIBO systems for filter maintenance, ensuring that all contaminated media are securely contained and disposed of without exposure risk.
Negative pressure environments and airlock entry systems to prevent the spread of airborne contaminants.
Validated cleaning and decontamination protocols for all equipment and surfaces in contact with potent compounds.
Real-time environmental and exposure monitoring to ensure that airborne concentrations remain within acceptable OEB thresholds.
Compliance with OEB safety standards is confirmed through rigorous risk assessments, performance qualification, and ongoing validation. International standards such as ISPE Good Practice Guide, ICH Q9 Quality Risk Management, and ISO 45001 Occupational Health and Safety Management Systems provide the framework for designing and maintaining compliant containment strategies.
Track Record
Explore our Gallery of Triumphs: Dive into our selection of recent success projects, where our focused approach and expertise have led to successful outcomes for our clients. Each case is a testament to our commitment to providing solid and effective solutions.

About QUALIA
QUALIA is comprised of a dedicated ensemble of 42 experts in the realms of pharmaceutical and biosecurity engineering. Our profound expertise encompasses design, construction, management, alongside both local and global consulting and validation services. We are steadfast in delivering comprehensive solutions tailored to biosecurity clean system engineering. Our portfolio includes a diverse assortment of products such as purification devices, biosecurity arrangements, HVAC systems, automation systems, validation services, process apparatus, and sterile piping. We take pride in our ability to devise proprietary products and systems that specifically cater to the nuanced demands of our clientele in the pharmaceutical and biosecurity industries.
Frequently Asked Questions
How to select the right cleanroom equipment for pharmaceutical manufacturing?
When selecting cleanroom equipment for pharmaceutical manufacturing, consider three critical factors: required cleanliness classification (ISO 5-8), process-specific containment needs, and regulatory compliance requirements. Evaluate equipment based on material compatibility with cleaning agents, maintenance accessibility, and validation documentation. Our biopharmaceutical specialists recommend conducting a comprehensive risk assessment to identify potential contamination sources before finalizing equipment specifications.
What are the key differences between BIBO systems and traditional filter housing?
BIBO (Bag In Bag Out) systems provide superior containment during filter changeouts compared to traditional housing by utilizing a sealed bagging system that prevents direct contact with contaminated filters. This significantly reduces operator exposure to hazardous materials and maintains cleanroom integrity during maintenance. Traditional filter housings require environmental shutdown during changes, while BIBO systems allow for continuous operation, improving efficiency and reducing production downtime.
How often should cleanroom equipment be revalidated?
Biopharmaceutical cleanroom equipment should undergo revalidation according to a risk-based schedule, typically annually for critical systems and after any significant modifications. Continuous monitoring systems can provide data-driven insights to optimize revalidation frequency based on actual performance rather than arbitrary timelines. Regulatory requirements may specify minimum revalidation intervals depending on your specific processes and product risk profiles.
What containment level is appropriate for OEB 4 compounds?
OEB 4 compounds require comprehensive containment solutions including closed systems, negative pressure environments, and redundant barrier technologies. Appropriate containment includes dedicated BIBO filtration systems, airlocks with interlocking door mechanisms, and continuous monitoring of differential pressures. Personnel working with OEB 4 materials should utilize proper PPE and follow strict standard operating procedures for material handling and decontamination protocols.
How do BSL-3 and BSL-4 laboratory requirements differ for containment equipment?
BSL-4 laboratories require significantly more stringent containment measures than BSL-3, including Class III biological safety cabinets or positive pressure suits with dedicated air supplies. Both require HEPA filtration for exhaust air, but BSL-4 demands redundant filtration systems with additional validation requirements. BSL-4 facilities must implement complete facility decontamination capabilities, while BSL-3 may utilize localized decontamination procedures based on risk assessment.